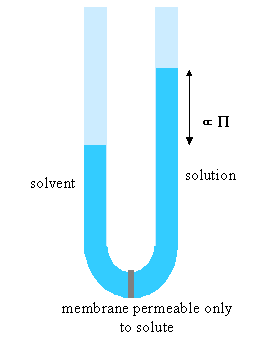
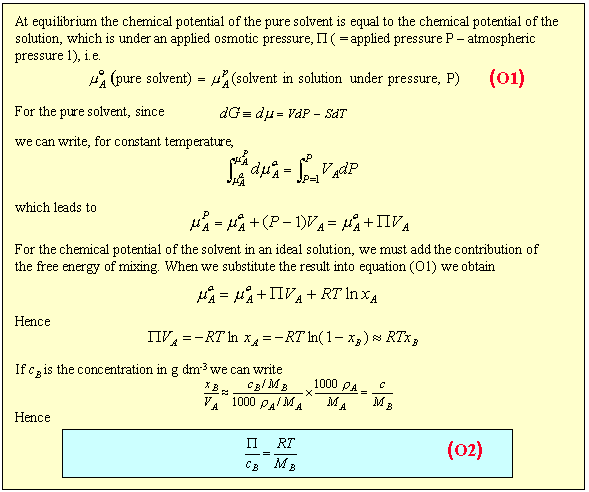
Of the properties so far described, osmotic pressure is the most useful. It is often used for the determination of molecular weights of large molecules such as polymers and proteins. The principle is shown in the diagram below. The pure solvent and solution are separated by a membrane permeable only to the small solvent molecules (the polymer cannot pass through). The chemical potential of the solvent is lower in the solution and it will therefore tend to pass through the membrane from pure solvent to solution. This process can be opposed by applying a pressure (additional to atmospheric and called the osmotic pressure) to the solution. The relationship of the osmotic pressure to the concentration and molecular weight of the solute is derived in the panel below the diagram.

In the case of polymer solutions the very large difference in size between polymer and solvent molecules usually causes the behaviour of the osmotic pressure to deviate from ideality. Equation (O2) then has to be replaced by equation (O3) below, in which B is a constant.
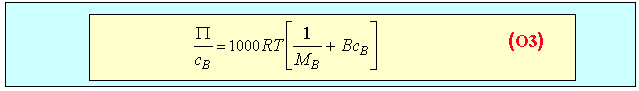
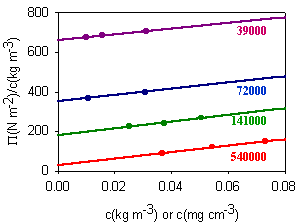
When the solution is not ideal the osmotic pressure must be measured at several concentrations and then a plot of P/cB against cB gives a straight line with intercept RT/MB at cB = 0, from which the molecular weight of B can be determined. Examples of such plots are shown in the diagram below for solutions of different molecular weight samples of the polymer neoprene in toluene.